Simple 1:1 Dose Conversion1
Dosing
A therapeutic dose on day 1 with the simplicity of no required titration1
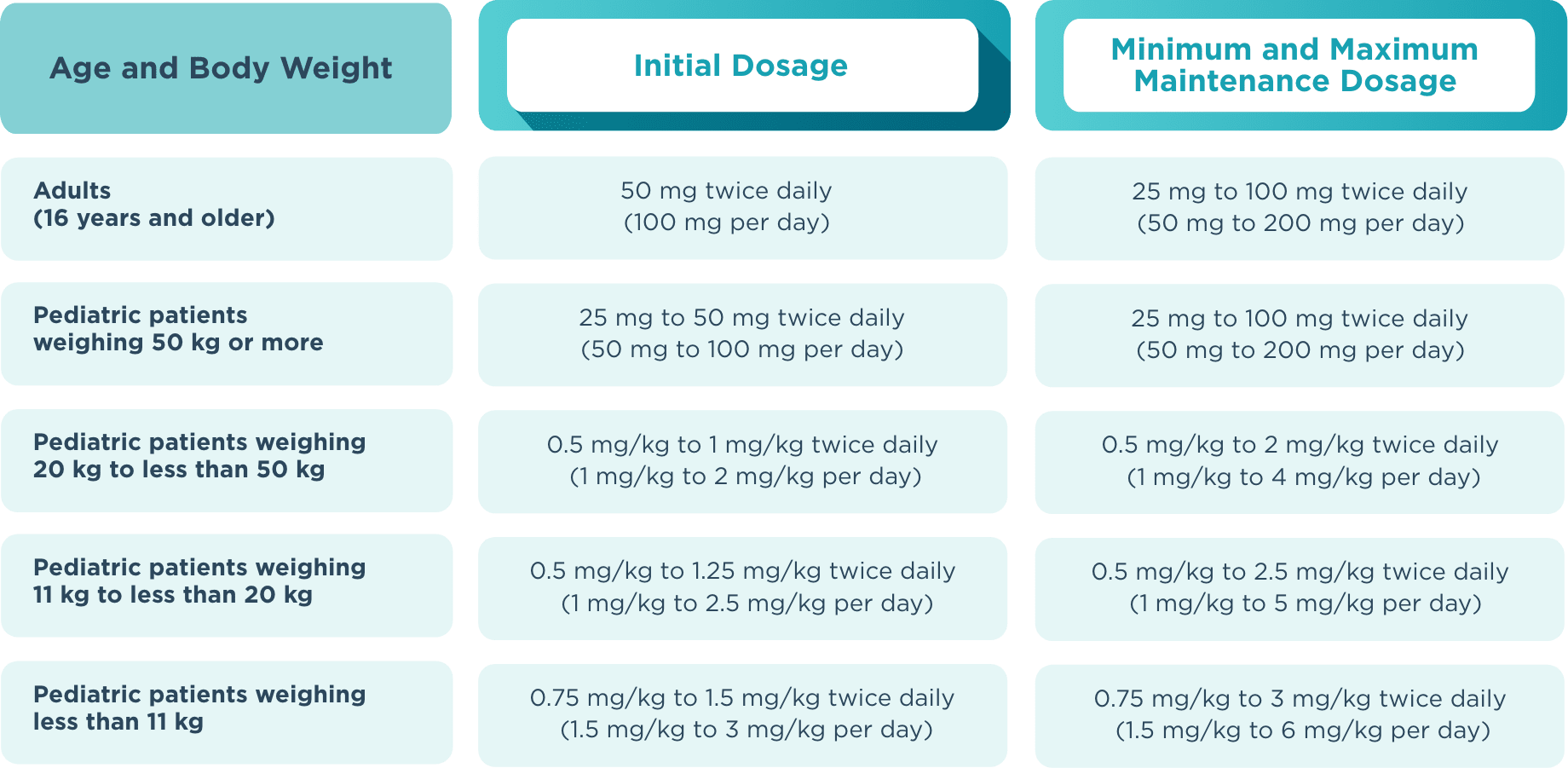
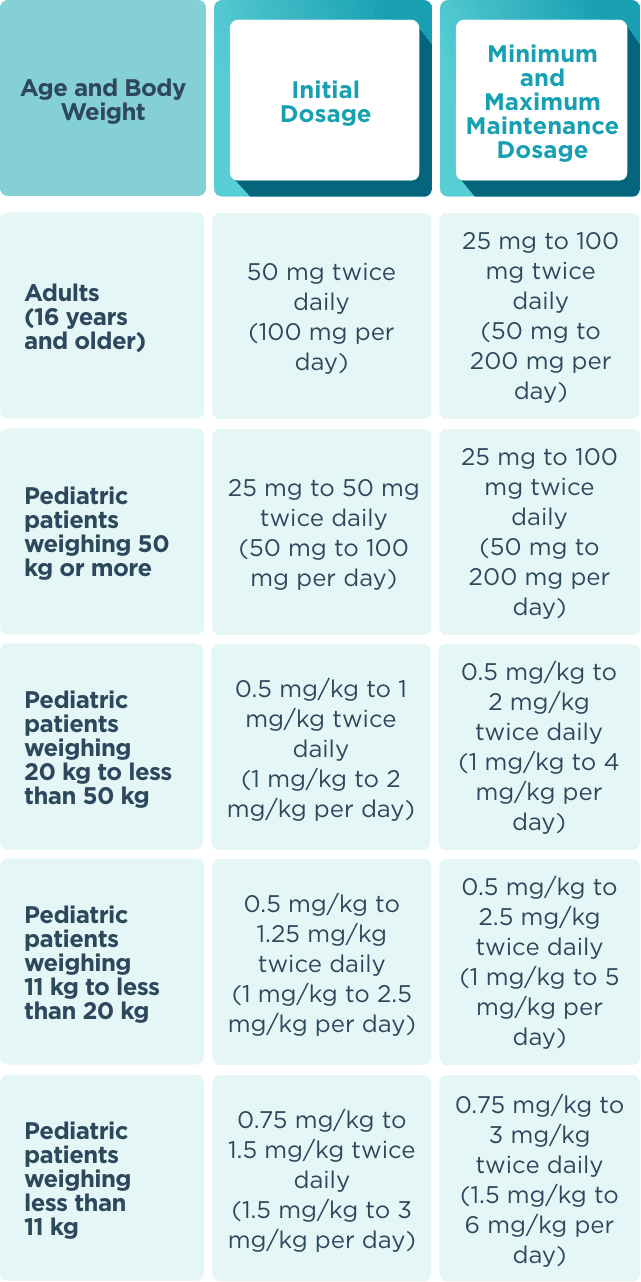
The recommended starting dose for monotherapy and adjunctive therapy in1:
- Adult patients (16 years or older) is 50 mg twice daily (100 mg/day)
- Pediatric patients weighing less than 50 kg is dependent on body weight
When initiating treatment, gradual dose escalation is not required. Dosage should be adjusted based on clinical response and tolerability.1
Recommended dosage and dose adjustments
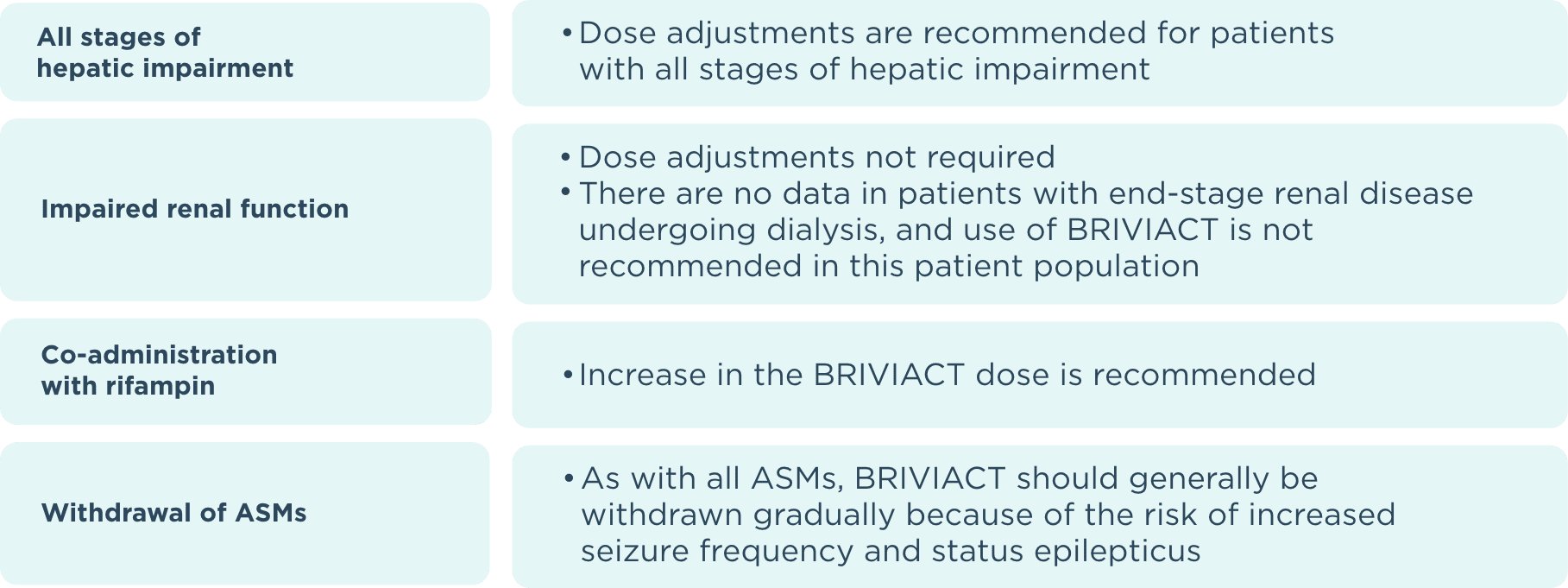
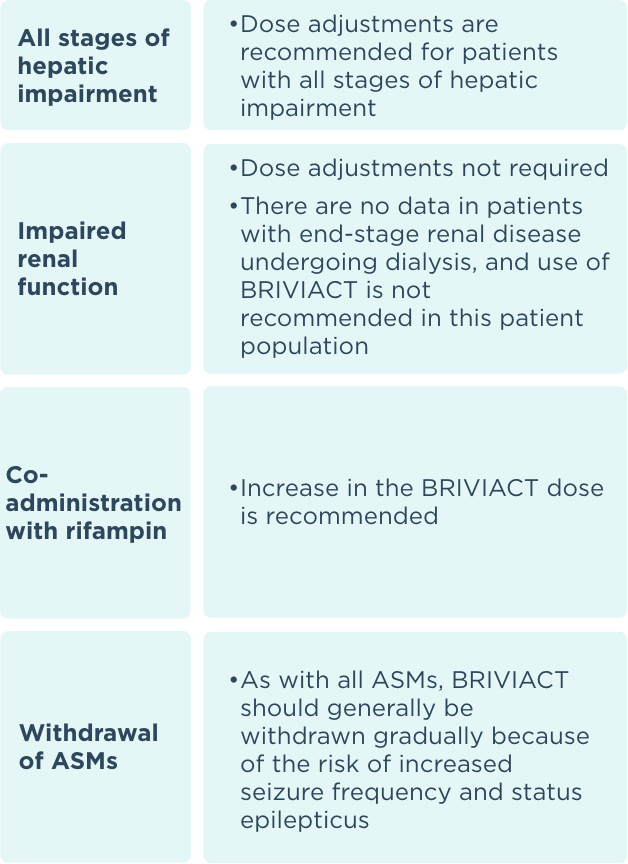
ASM=antiseizure medication.
Formulations
Multiple formulations offer flexible administration1
Simple 1:1 dose conversion

Oral solution 10 mg/mL:
300-mL bottles
Oral solution has a shelf
life of 5 months after
opening
Tablets*:
60-count bottles
*10-mg tablets are also available.
Injection solution
50 mg/5 mL:
Single-use vial undiluted
injection or infusion
Products not shown at actual size.
Can be given with or without food
Tablets should be swallowed whole with liquid. They should not be chewed or crushed
No blood level, respiratory, or cardiac monitoring required
No refrigeration
required
Intravenous injection only1
- BRIVIACT injection may be used when oral administration is temporarily not feasible
- BRIVIACT injection should be administered intravenously at the same dosage and same
frequency as BRIVIACT tablets and oral solution
BRIVIACT injection offers rapid administration1
Injection should be administered intravenously over 2 to 15 minutes. Clinical study experience is up to 4 consecutive days of treatment
No dilution required
Reference: 1. BRIVIACT® (brivaracetam) prescribing information. Smyrna, GA: UCB, Inc.